Intranasal Naloxone for acute opiate overdose: Reducing needle stick risk, improving time to medication delivery
Table of Contents:
Introduction to intranasal naloxone (click here)
Reducing needle stick risk in the prehospital environment (click here)
Literature overview and discussion
Lay person naloxone home treatment (click here)
Paramedic use of intra-nasal naloxone (click here)
Personal insights from experienced clinicians (click here)
Treatment protocol (click here)
Teaching Materials (click here)
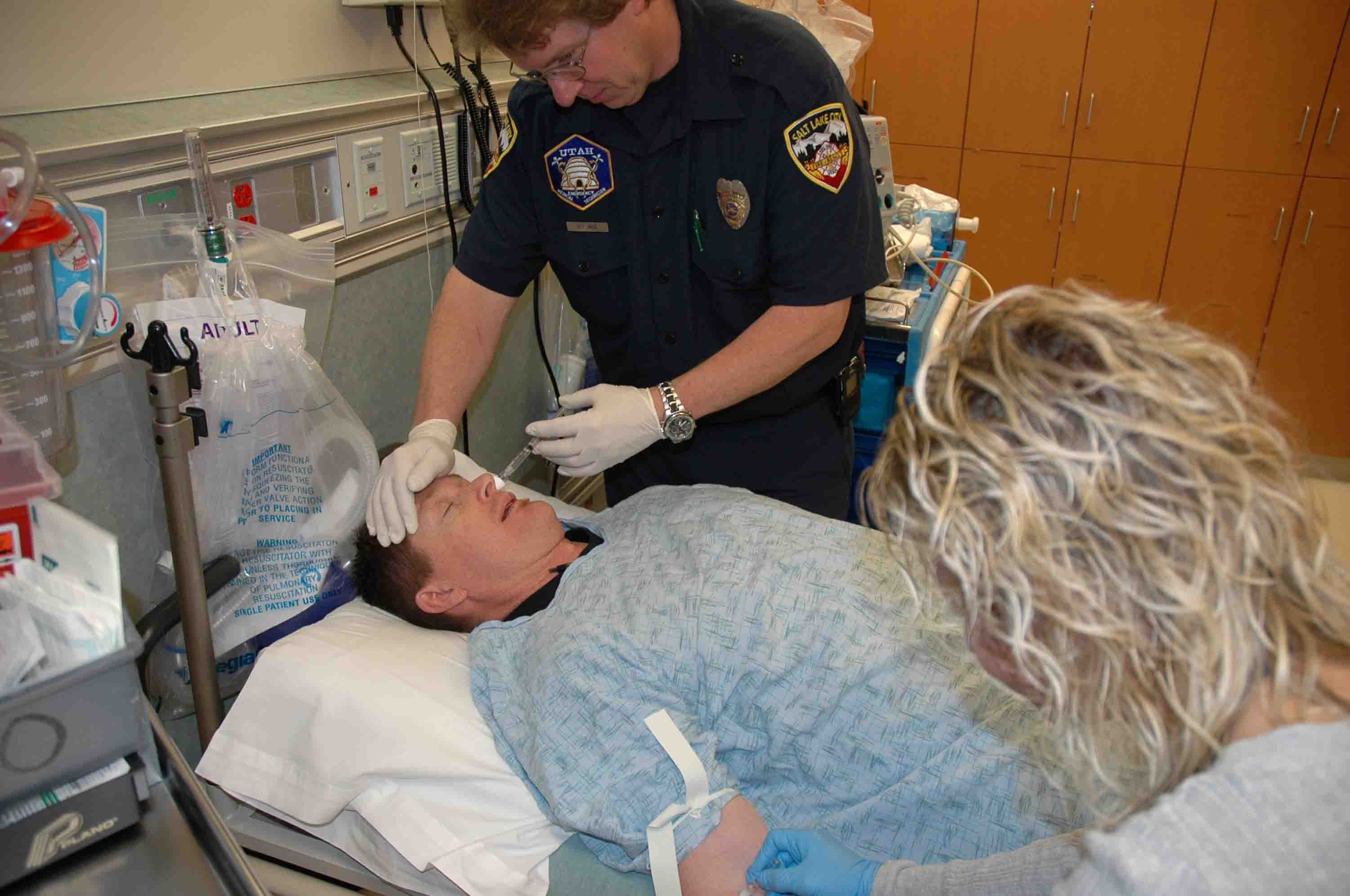
Delivering intranasal naloxone to a patient suffering from an opiate overdose
W.H.O. (World Health Organization) 2014 recommendations regarding Community management of opioid overdose - download the PDF
Introduction
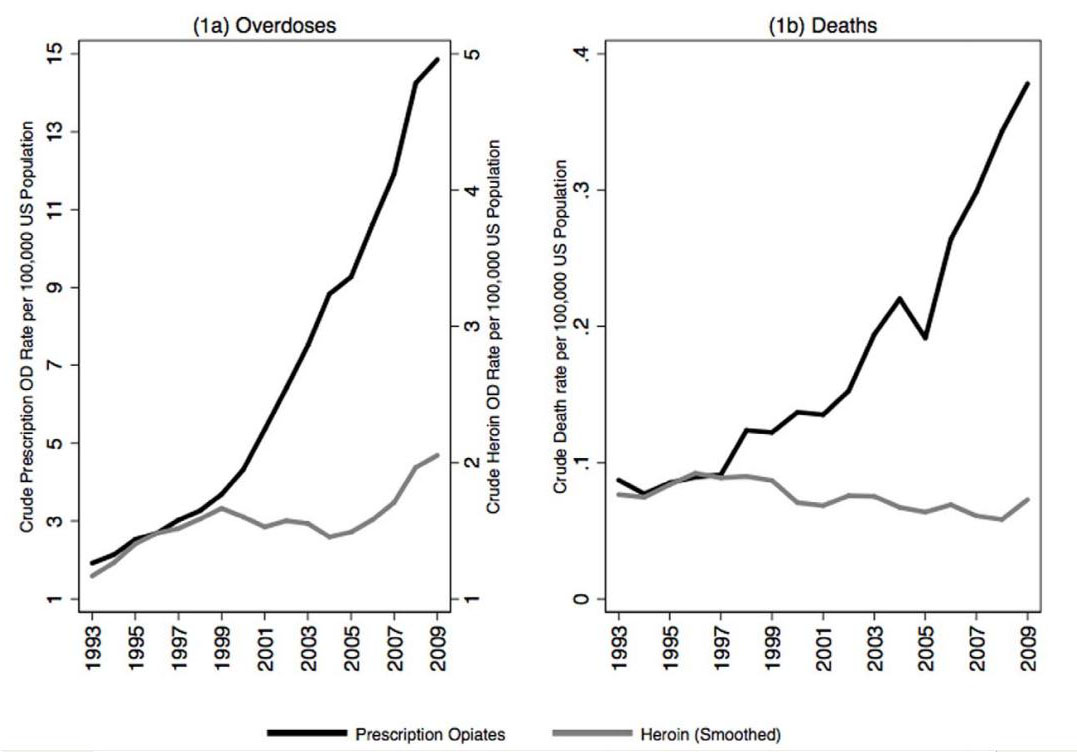
There is currently a world wide epidemic of opiate overdoses and deaths due to accidental opiate overdose which is especially apparent in the United States. The majority of those deaths are related not to heroin, but instead to prescription drugs. This diagram from Unik et al points to the dramatic rise that has occurred over the last 15 years.[28] (Click here for full article link)
Furthermore, Intravenous drug users (IVDUs) requiring naloxone after heroin overdose are a unique population that place prehospital health care providers (paramedics/EMTs and other ambulance personnel) at an especially high risk for blood borne pathogen exposure.[1-3] Since all of these patients rarely need intravenous access for any reason beyond the administration of naloxone (Narcan), a method of administering naloxone without a needle would be preferable.[4-6] Fortunately, naloxone is a small molecule that easily crosses the nasal mucosal membranes. After intranasal (IN) administration, naloxone exhibits opiate antagonist effects almost as rapidly as the IV route with bioavailability approaching 100%.[7, 8] Based on this information two compelling reasons exist to consider IN delivery of naloxone for acute opiate overdoses: The reduction of needle stick risk to rescue providers and the possibility of lay person naloxone delivery.
Reducing needle stick risk in the prehospital environment:
While the intranasal option for delivering naloxone is not necessarily more effective than traditional intramuscular or intravenous injection methods, it is easier to deliver and often works as well as an injection. Most importantly to health care workers, intranasal naloxone delivery eliminates the risk of a contaminated needle stick. Needle stick injury is not a minor issue. Blood borne exposures are an occupational hazard that healthcare providers face daily. The CDC estimates that 600,000-800,000 percutaneous injuries with contaminated sharps occur yearly in the United States.[9] With the increasing prevalence of blood born pathogens such as human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) accidental needle stick injury may pose a life-changing and possibly life-ending event for affected health care workers. This risk is higher in the prehospital environment where a combination of patient and environmental factors make needle stick injury more likely.[10] Marcus et al found an HIV seroprevalence rate of 4.1 to 8.9 per 100 patient visits in three inner-city ED populations.[1] Because the annual blood contact for an individual EMS worker (Emergency Medical Services – paramedic) has been estimated to be as high as 12.3 per year, concern exists regarding the risk of viral seroconversion in EMS providers.[11] Several authors have validated this concern. Valenzuela et al reported a five-fold higher prevalence of Hepatitis B (HBV) infection in paramedics than that observed in a comparable population from the same city.[12] Pepe et al noted a strong association between years of employment and the rate of HBV infections in EMS workers.[13] Although there is less risk today with the advent of HBV vaccines and use of universal precautions, the risk for other exposures remains significant.
An especially high-risk patient population to EMS providers is the IVDU. These patients have HIV, HBV and Hepatitis C (HBC) seroprevalence rates that are far higher than the baseline population.[2] In addition, EMS personnel commonly are involved in their care for life threatening illnesses such as respiratory arrest from opiate overdose. Furthermore, unique EMS environmental conditions such as combative patients, uncontrolled scene issues, poor lighting and moving ambulances make the probability of suffering a needle stick even more likely than in more controlled medical settings. Since opiate overdose patients rarely need an IV for any reason beyond the administration of naloxone, a needleless method of administering naloxone would eliminate needle stick risk and potential transmission of blood borne pathogens.[4-6] Effective methods of reducing needles stick risk to emergency providers in this situation should be welcomed. Intra-nasal naloxone is one such therapeutic intervention that may have a role in opiate toxic patients.[14, 15] The literature review that follows will discuss the results of currently published trials investigating IN naloxone in the prehospital environment.
Literature overview and discussion
Lay person and BLS naloxone treatment
Trials utilizing home injections of naloxone for heroin overdoses have demonstrated some success, but routine application of this concept is limited by the need for injection training and by state laws.[16] These issues have led some investigators to consider intranasal naloxone since it can be delivered without a needle very easily by the lay public including family members, law enforcement and first aid workers.[17] There is no need to learn 1) How to administer an injection, 2) Sterile technique methods, or 3) Intravenous cannulation or injection techniques. In addition, administration by the nasal route may be more appealing to IVDU who fear needles. Finally the risk of needle stick injury will be eliminated during administration. Many states have now adopted home layperson administered intranasal naloxone into their state regulations.[18, 22]
As of 2014 the clinical need for layperson administered naloxone has become overwhelming due to evidence of its efficacy (see data below) and the epidemic of opiate induced deaths that have occurred world wide but especially in the USA where 80% of all prescription opioids are consumed. This had led to a plethora of new laws approving layperson and BLS administered naloxone and great deal of literature on the topic.
Massachusetts, specifically the Boston area, has
been one of the ground breaking areas in terms of advancing the cause of
expanded naloxone access to basic life support personnel (Firefighters,
EMT basics, police) and the lay public. They have also led the country
in academic level research on the topic. In 2014 they provided a
plethora of new insights into this topic.
From their EMS agency we get a large review of experience with BLS level data from 2006-2012. Weiner et al conducted a retrospective review of 7 years data where the city of Boston BLS providers administered intranasal naloxone to suspected opiate overdose patients.[32] A total of 793 uses were identified and 724 charts were found to match with ED records. 689 (95.2%) of patients given naloxone by BLS providers responded to the drug. Only 8.8% required another dose in the ED. 507 (70%) were discharged home. The authors conclude that out-of-hospital administration of BLS naloxone was effective in the vast majority of opiate overdose cases and only a small percentage of patients required additional ED interventions. (In 2013 the BLS service administered another 458 doses of naloxone in the field).[33] Davis et al provide additional insights into BLS administered (firefighter and police first responders) naloxone in the area surrounding Boston.[33][Free open access article click here] These communities already have demonstrable reductions in fatalities from opiate overdoses due to lay public administered naloxone and now have another layer of safety introduced to intervene in the current epidemic of opiate induced deaths.
Other communities are now showing promising results from naloxone
programs. Rando et al report historical data on a community’s
death rate from opioid overdose
(Lorraine OH) before and after introduction of police administered nasal
naloxone. [50] The death rate per
quarter increased from 5.5 in 2011 to 15.3 for 2012 and peaked at 16.3
for 2013. After introduction of police officer delivered naloxone the
death rate receded to 13.4 over the next year.
Does naloxone distribution increase risky behavior? The answer is no - in fact the opposite is more likely:
Researchers from Boston also have addressed the concern by some
that naloxone distribution may result in increased risky behavior by
opiate users. Doe-Simkins et al
review the data concerning this issue and provide their own additional
experience in an article from Biomed Central.[34]
[open
access – click here for full article] Part of this concern
regarding increasing use of
heroin due to providing an antidote stems from a survey conducted by
Seal et al. in 2003. [35] These
authors interviewed 84 injection drug users and were told by 35% of
respondents that if they were provided with a naloxone rescue kit they
might be more comfortable using heroin more often. However, this opinion
was refuted by actual evidence when these same authors trained 12
pairs of injection drug users (24 people) to perform CPR and to give
naloxone on the street.[36] In the
next 6 months these individuals reported seeing 20 overdoses for which
the performed CPR and/or gave naloxone in 19 (95%). All victims
survived. The “providers” trained to do these interventions actually
began using less heroin, not more heroin. Similar findings are reported
by Wagner in a 2010 article.[37] In a 2014 article Wagner reports on interviews conducted with a
cohort of active injection users who were trained to use naloxone and
actually found that these trained drug users moved up in the social
strata of their community due to the lifesaving skill they acquired.[38]
This resulted in greater feelings of self-worth. The majority of those
trained reported using less heroin and some actually left this social
group to reduce their exposure to this risky behavior.[open
access data – click here for articles - Wagner article is at the end of
the series] Galea’s data from 2006 and Doe-Simkins data from
2014 also showed no increase drug use following the introduction of
rescue naloxone kits. [34, 39]
In 2014 there has been a great deal of activity on
this topic throughout the USA with multiple state legislatures recently
approving layperson and BLS use of naloxone, pharmacists pushing for
increased ability to provide this therapy and emergency physicians
addressing this need at a national level.
Bailey and Wermeling advocate for
a greater role of pharmacists in identifying high risk patients (licit
users of high-dose prescription opioids or injection drug users and
abusers of prescription medications) and either contacting their
provider for a prescription for IN or IM naloxone or if legal,
prescribing the naloxone themselves (with appropriate training for
use).[40] Hammet et al further elucidate
the legal aspects related to this.[45]
The American College of Emergency Physicians (ACEP) revisited the
concept of expanded access to naloxone at a BLS and lay public level and
the concept of physician prescribed naloxone to high risk patients. As a
point of history, these proposals were rejected by the council in 2013,
but resurfaced at their national meeting in Chicago during the month of
October 2014. Both proposals have now been passed, garnering support for
these policies from yet another important player involved in the care of
this illness.
Both Maryland and Rhode Island
are moving forward with educational programs to expand access to
naloxone.[41]
Additional reports of layperson administered naloxone are surfacing from
around the world.
Green et al describe two cases of opioid overdose reversal in former
prisoners who were trained to use IN naloxone upon prison release. In
both cases the patients were able to assist their partners in
giving them the naloxone and reversing their overdose. The authors
advocate training and equipping former prisoners and all at risk
patients and families of these patients.
I
Layperson administer naloxone kits:
One ampule of naloxone 2 mg/2ml
One Luer attached atomizer

The state of New Mexico allows both their basic life support [BLS] providers (police and highway patrol) to administer IN naloxone and they send IN delivery kits home with families of known opiate addicts in an attempt to reduce the high rate of opiate overdose deaths in their state.[18] Similar community efforts are now ongoing in multiple states including Massachusetts (NOMAD program), New York and North Carolina (Project Lazarus). Maya Doe-Simkins published preliminary data form the greater Boston area experience with lay person administered intranasal naloxone, noting a total 385 participants trained and 74 successful opiate overdose reversals - leading to reduced EMS and ER utilization and likely reduced mortality.[22] (Click here for the article) . Since the publication of the article, the system (INPEDE OD study - N.O.M.A.D. program) now reports 755 opioid overdose reversals with that number growing daily. (click here for 2011 report).
The February 17, 2012 Morbidity and Mortality Weekly Report (MMWR) published by the CDC discusses the community based opioid overdose prevention programs that exist in the USA, most of which pass out naloxone as either intranasal or intramuscular forms of delivery. (Click here for a link). This report states that over 53,000 laypersons have been trained with a reported successful reversal of over 10,000 patients who have overdosed. At least 15 states have existing programs. This report was featured in Time magazine (click here for link) where the authors suggest this should be made an over the counter therapy (another link on this here) and suggest that the FDA will be considering this in the spring of 2012.
Massachusetts N.O.M.A.D. program (Not One More Anonymous Death overdose prevention project)
As described above the NOMAD program has been quite successful using layperson administered intranasal naloxone combined with rescue breathing until the naloxone has had a chance to work. As of September 2011 they report over 1000 successful overdose reversals.
Here is a link to the protocol / photos of what the NOMAD program teaches the lay public:
N.O.M.A.D. Not One More Anonymous Death overdose prevention project protocol (Click Here)
The same group who was involved in the NOMAD program established an Overdose education and naloxone distribution (OEND) program for patients in Massachusetts who were being started on methadone.[27] They distributed kits to 1553 new methadone maintenance patients and reported 92 naloxone rescues as a result. Interestingly, though prescription opiates are now more commonly associated with death than heroin, in this group of methadone patients, the vast majority of the naloxone rescues occurred on witnessed heroin overdoses.
New York City also reports approximately 300 opiate reversals and growing using a similar program.
Intranasal and intramuscular naloxone program in New York City - Power Point - (click here)
CVS pharmacy kits:
As of the fall of 2015, CVS pharmacy now sells naloxone kits over the counter in 14 states: Those states include Arkansas, California, Minnesota, Mississippi, Montana, New Jersey, North Dakota, Pennsylvania, South Carolina, Tennessee, Utah, and Wisconsin. Naloxone is already available over the counter at CVS stores in Rhode Island and Massachusetts.
http://www.theverge.com/2015/9/25/9398823/naloxone-heroin-overdose-cvs-stores-over-the-counter
http://www.huffingtonpost.com/entry/cvs-naloxone-overdose-reversal_5602dba2e4b0fde8b0d0d189
New York City nasal naloxone device:

New commercially available Nasal Naloxone
In 2019 Krieter et al present initial pharmacokinetic data regarding the newly available prepackaged IN naloxone product marketed by Aptar. Bottom line is that IN naloxone (4 mg) results in higher plasma concentrations of drug than the intramuscular dose (0.4 mg) yet does not require any injection. They also found that over 90% of the lay public could use the product with no prior training. [68, 74]
Other commercially available nasal naloxone products are entering the market.[71] All are highly concentrated and therefore should improve efficacy following nasal delivery. Maybe the competition will lead to a fair price
If these products are priced at an affordable cost, I predict it will displace all other forms and could become a huge commercial success going home with everyone on long term opiates. However, given recent (2016) obvious greed and over-pricing of life saving home medications such as Epi Pens, I will believe this when I see it.
Availability and cost of nasal naloxone in pharmacies and to hospitals:
Unfortunately the trend, as with many pharmaceuticals now, is an upward push on prices for any drug that is controlled by a single manufacturer. Rosenberg reports a similar trend for all forms of naloxone.[64]
In 2017 researchers found that
only 1/3 of Philadelphia pharmacies carried nasal naloxone and 1/3 of
those required a prescription to obtain it – this despite a law passed
two years earlier allowing pharmacies to stock and dispense the drug
without any prescription. To make matters worse pharmacies in the areas
with the highest opiate overdose rates had the lowest naloxone
availability. Drug costs were a mean of $145 ($119-150).
In
contrast to Philadelphia, Wu et al surveyed Massachusetts pharmacies and
found 98% stocked the drug and 96% did not require a prescription. Costs
however were similar with mean price being $128.
Others confirm the regional variability in access to this life saving medication.[72, 73]
Blurb on the tragic costs of naloxone:
https://www.statnews.com/2018/11/08/costs-heroin-naloxone-tragic-snapshot-opioid-crisis/
Given the concern of
skyrocketing costs and drug shortages, Pruyn et al sought to determine
if expired naloxone vials contained drug that was still effective.[70]
They tested samples of naloxone with expiration dates ranging from 1990
to 2018. They found that most samples still contained over 90% naloxone
(not is breakdown product) – even samples from the 1990’s. Though there
was a slight degradation correlating with time they found no significant
degradation that would effect the drugs use. The conclude that
“Extending the shelf-life of naloxone products may have important
financial and public health consequences in addressing future drug
shortages and meeting the needs for this critical drug.”
Editorial comment: Naloxone
prices have skyrocketed in the last 20 years. (The opposite of
traditional supply/demand economics because our pharmaceutical industry
does not lie within a traditional economic system). One solution, minor
though it is, might be to use expired drug if it is still effective.
Pruyn showed that to be the case implying that if you have stored your
naloxone out of the light and heat it is likely still fine. Test a vial
– if it works – maybe you should use it. I know for a fact (having been
in the industry) that expiration dates in medical supplies are set based
almost entirely on time required to distribute the product to the shelf
with an expiration date of about 6-12 months or so later. Expiration
dates for these non-spoilable product have almost nothing to do with the
actual shelf life of a product. The
medical industry generally does not test to see how LONG their product
will last, they only test to be sure it can last to the date of delivery
plus 6-12 months beyond.
BLS provider administration of IN naloxone
Multiple states and city's allow IN naloxone delivery by lay persons so it makes sense that they allow their BLS providers to also administer this potentially lifesaving medication. In 2005 Boston EMS approved IN naloxone for their EMS providers. In 2006 they reported a 75% success rate in reversal of opioid overdose when BLS providers delivered IN naloxone. (Click here for a slide presentation on the topic.) In a more complete 5 years study they found IN naloxone delivered by BLS to be 70% effective with rare incidence of acute agitation. they did find it to seem to be slower than injectable naloxone (which is similar to Kerr data from Australia) - not perfect but no need for a shot. (Click here for slide presentation for Boston 2005-2009 BLS data).
Coffin and Sullivan used all the above data along with
many other studies related to home use of injectable naloxone and did a
cost analysis of the utility and expense related to home naloxone
therapy.[29] Although this is a very statistically complex article, it
assesses a very important issue relating to layperson delivery of
naloxone (whether via IN or IM delivery). The authors cite a common
concept used to determine the value of a medical intervention in terms
of costs per year of additional life – the concepts is called “quality
adjusted life years” or
QALY. Researchers and public health officials commonly use a value of
$50,000 per year of life gained as a cost effective number when
calculating QAKY. In other words – if you can
provide a therapy such as cancer care, organ transplantation or
delivery of naloxone for a cost of $50,000 or less per additional year
of life it is considered cost effective medicine.
Using this cut off as the maximum, the authors then utilized all
the published literature available on the topic of heroin overdose,
death rates, etc and made some assumptions both at the low and high end
of costs with an assumption that an overdose results in death only 1% of
the time, that only 13.6% of distributed naloxone will be used in a year
and that naloxone kits cost between $15 and $30. They found that the
cost for one QALY was $438. On the outside using the assumption that a
heroin user who does not die costs society more than they provide – the
outside highest QALY was $2429. Using the absolute worst case scenario
where overdoses were rarely witnessed and naloxone was rarely used,
barely effective and very expensive they still found on QALY to be
$14,000. They hypothesize similar cost effectiveness for use of naloxone
to reverse accidental overdose from prescription opiates (which is now
the leading cause of death in young adults in the United States.
In an accompanying editorial, Compton et al
[30] review the FDA views on the topic – which are extremely favorable
towards prescription home naloxone and eventually over the counter
naloxone. At this point the FDA is fully aware of the off-label lay
person use of naloxone both nasally and Intramuscularly and applauds
this movement but hopes to encourage the pharmaceutical industry to
develop easily administered and more highly controlled forms of this
medication.
Editorial
comment: Is a year of your life worth $438-$14,000? Is it worth saving
someone else's life – often a young adult -
so they can go on to experience life for decades to come? I doubt
this is a very hard decision and I find it amazing and sad when I hear
some clinicians or politicians comment on the ethics of distributing
naloxone to lay people and their concern that it will increase risk
taking. T
In 2016 Fisher et al published data demonstrating that non-medical first
responders (police) are fully capable or delivering IN naloxone to
appropriate candidates.[54] The data is essentially identical to EMS
data - noting over 80%
effective response to the drug (breathing). Only one case (out of 126
patients) led to combativeness – also confirming the low incidence of
agitation following IN naloxone delivery.
Conclusions regarding layperson and BLS administered naloxone
The evidence increasingly supports the effectiveness and safety of layperson administered naloxone. Furthermore, the USA is in the throngs of a major epidemic of opiate overdose deaths from prescription pain medications with some states death rates exceeding that of motor vehicle crashes, homicides, and many other causes of death in young adults. Hopefully we will see the FDA make this a non-prescription therapy in the very near future.
Paramedic use of intra-nasal naloxone
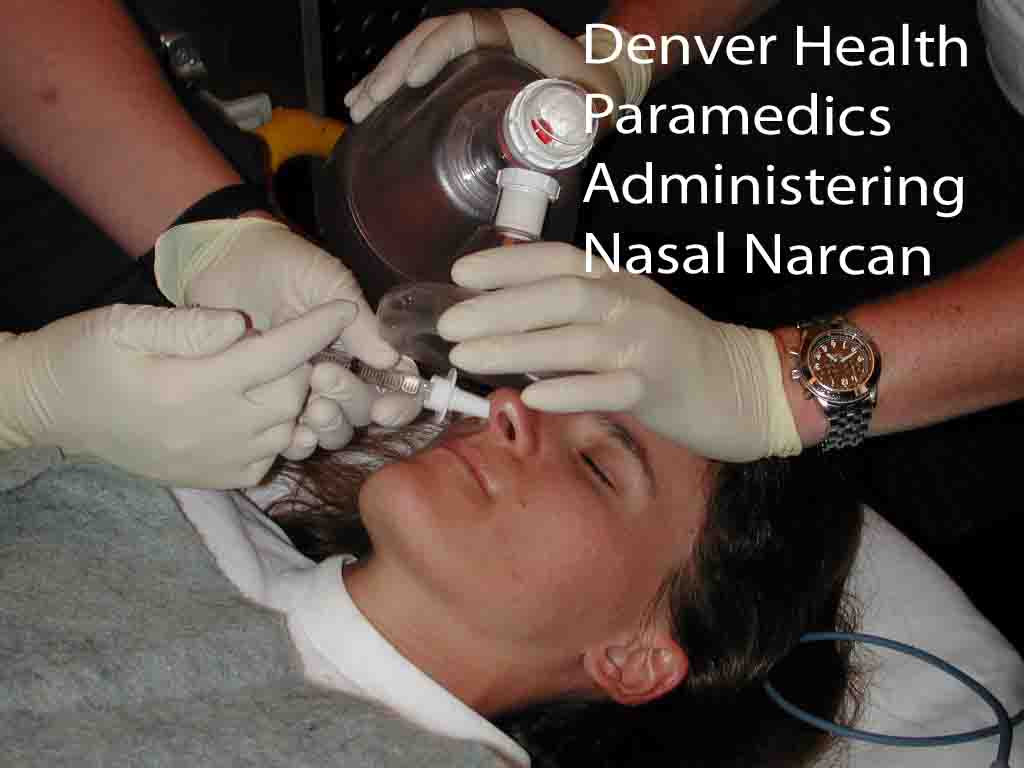 The
Denver Health Paramedic system investigated the efficacy and safety of
atomized intranasal naloxone for the treatment of suspected opiate
overdose.[14] Study patients were given 2 mg of IN naloxone (1mg/ml up
each nostril) upon initial contact. After intranasal naloxone, standard
protocols were followed including airway management, IV placement, and
administration of IV naloxone. Ninety-five patients were enrolled.
Fifty-two patients responded to naloxone: 43 (83%) to IN naloxone alone,
9 (17%) to IV following IN naloxone. Four of these "non-responders" had
IV naloxone so rapidly (less than 3 minutes) that it is likely the nasal
naloxone did not have time to produce a clinical effect. An additional
four of the nine "non-responders" had anatomic abnormalities that may
have prevented intranasal medication absorption (epistaxis, nasal
trauma, nasal septal abnormalities). The median times from arrival at
patient side to awakening and from administration of the IN naloxone to
patient awakening were 8.0 minutes and 3.0 minutes respectively. These
median times to awakening after arrival and naloxone administration are
less than those reported by Wanger et al for intravenous naloxone (9.3
minutes and 3.8 minutes) or subcutaneous naloxone (9.6 minutes and 5.5
minutes).[19] Even though this was a limited study, the
authors concluded that IN naloxone can be effective in the field (83%
initial response rate), acts rapidly and could potentially reduce the
risk of paramedic needle sticks in this population.
The
Denver Health Paramedic system investigated the efficacy and safety of
atomized intranasal naloxone for the treatment of suspected opiate
overdose.[14] Study patients were given 2 mg of IN naloxone (1mg/ml up
each nostril) upon initial contact. After intranasal naloxone, standard
protocols were followed including airway management, IV placement, and
administration of IV naloxone. Ninety-five patients were enrolled.
Fifty-two patients responded to naloxone: 43 (83%) to IN naloxone alone,
9 (17%) to IV following IN naloxone. Four of these "non-responders" had
IV naloxone so rapidly (less than 3 minutes) that it is likely the nasal
naloxone did not have time to produce a clinical effect. An additional
four of the nine "non-responders" had anatomic abnormalities that may
have prevented intranasal medication absorption (epistaxis, nasal
trauma, nasal septal abnormalities). The median times from arrival at
patient side to awakening and from administration of the IN naloxone to
patient awakening were 8.0 minutes and 3.0 minutes respectively. These
median times to awakening after arrival and naloxone administration are
less than those reported by Wanger et al for intravenous naloxone (9.3
minutes and 3.8 minutes) or subcutaneous naloxone (9.6 minutes and 5.5
minutes).[19] Even though this was a limited study, the
authors concluded that IN naloxone can be effective in the field (83%
initial response rate), acts rapidly and could potentially reduce the
risk of paramedic needle sticks in this population.
Kelly et al conducted a similar EMS study, comparing intranasal naloxone to intramuscular naloxone in 155 prehospital opiate overdose cases.[15] Unfortunately they did not have access to concentrated naloxone and had to use 2 mg of naloxone in 5 ml of solution – a volume that would be predicted to be less effective due to run-off into the throat (See intranasal medication delivery overview section of this web site). Nevertheless, they still found both treatments equivalent in terms of opiate reversal (74-83%) though IM naloxone worked faster. Interestingly, only 2% of patients given intranasal naloxone experienced agitation or irritation upon awakening, a difference they attributed to the gradual absorption and gradual awakening seen with intranasal naloxone. This finding was felt to be an advantage of IN naloxone, since the rapid awakening and hypoxic agitation seen with administration of IV naloxone is of considerable concern to some EMS providers. Based on this data and the considerable danger of needle stick exposure in this patient population, these authors conclude that IN naloxone should be the first line therapy for opiate overdose in the prehospital setting.
Robertson et al reviewed their EMS data on 154 opiate overdoses requiring rescue naloxone over a 17 month period.[21] They found that the EMS providers used IV naloxone 104 times and IN naloxone 50 times. The mean time from arrival at scene to awakening was identical for both delivery routes (about 20 minutes) though naloxone was faster in onset once an IV was established (8 minutes versus 12 minutes). 34% of patients in the naloxone grip were given a second dose, while 18% in the IV group needed a second dose. The authors conclude "Given the difficulty and potential hazards in obtaining IV access in many patients with narcotic overdose, IN naloxone appears to be a useful and potentially safer alternative."
Merlin et al did a retrospective review of all naloxone administration in their single 6 truck EMS system over a two year period.[23] They then eliminated all cases that did not have confirmed opiate overdose (admitted by patient or family, found with paraphernalia of opiate injection, confirmed by urine toxicology screen). Using this strict inclusion criteria they found 96 cases of opiate overdose treated with naloxone. Of these cases 55 received IV naloxone, 38 intranasal and 3 intramuscular. Comparing baseline respiratory rates and change in Glasgow coma scores they found no statistical difference: IV naloxone patients had increase in respirations from 10/minute to 18/minute and GCS increase from 4 to 15. IN naloxone patients had increase in respirations from 10/minute to 16/minute and GCS increase from 3 to 12. The authors conclude that "among subjects with confirmed opioid overdose, intranasal naloxone is as effective as intravenous naloxone at reversing central nervous system depressive effects caused by opioids."
A trial of concentrated intranasal naloxone [2mg Naloxone in 1mL] for suspected heroin overdose was done in the prehospital setting in Melbourne, Victoria. The findings were published in December
2009. They compared 172 patients randomized to IN versus IM naloxone. Response rates and times were the same after 1 dose (72% vs. 78%, 8.0 versus 7.9 minutes onset, p=NS). 18% of the IN patients were redosed for a total response rate of 82%. The authors conclude that intranasal naloxone is effective and safe and offers a needle-less method of treating heroin / opiate overdoses.[24] Other publications suggests that IN naloxone should move from the realm of ALS to that of BLS, allowing all first responders to administer this medication intranasally to any comatose patient at risk of opiate overdose.[17]McDermott demonstrated that paramedics felt IN naloxone was faster to deliver, better accepted and perceived as safer than IV naloxone and that this route should be considered more frequently as first line therapy.[26]
Sabzghabaee
et al conducted a prospective RCT comparing IN naloxone (0.4 mg) to IV
naloxone (0.4 mg) in 100 patients who overdosed on opiates.[31] All
patients were delivered the study drug and had their ventilation
supported for 5 minutes. After 5 minutes those who failed to respond
were administered a second dose – it is not clear from the paper how
many patients required redosing. The primary outcome was level of
consciousness with secondary outcomes of vital signs (primary interest
was respiratory rate), time to response, oxygen saturation and side
effects (agitation). They found both treatment regimens equivalent in
reversing both respiratory depression and CNS depression. 100% of the IN
group progressed to a state of either lethargy or full consciousness
following naloxone delivery compared to only 60% of the IV group (the
remaining 40% were obtunded and breathing but no longer comatose). The
time to response following drug delivery was 2.56 minutes for the nasal
route versus 1.48 minutes via the IV route. There was no difference in
respiratory rate improvement. Mean arterial saturations increased from
71% to 94% (IN) and 73% to 94% (IV). Agitation was observed in 12 of 50
patients receiving IV naloxone but in no patients receiving IN naloxone.
The authors conclude that IN naloxone is as effective as IV naloxone in
reversing both opiate induced respiratory depression and CNS depression,
but that the nasal form leads to less severe withdrawal symptoms
following delivery and is therefore preferred.
(Free
article – open access click here for PDF)
In 2019 Dietze et al published their findings on this
lower dose IN naloxone. They used a 0.8 mg in 1 ml naloxone formulation
available in Australia and compared its efficacy when delivered nasally
versus intramuscularly.[65] They randomized 197 patients.
They found the IM dose to work
within 10 minutes in 91% of patients (most studies using IM. IV or IN
find maximum efficacy in the 85-95% range so this is as good as you can
generally expect). The 0.8 mg dose nasally worked in 77% of patients – a
statistically and clinically important reduction in efficacy.
Comments: For 15 years or more I have been asked if it is ok to give the 0.4 mg/ml standard naloxone dose intranasally to treat opiate overdose. My answer was always to follow the literature – at the time only 2 mg/2ml had been studied so unless they were conducting a research project I felt using the 0.4 mg dose was risky as it was potentially too dilute to be effective. Admittedly Kelly et al published their results using this formulation in 2005 – but they gave 5 vials of the drug up the nose (5 ml) so surely had runoff, but did achieve a 2 mg dose. They found 74% of cases aroused compared to 83% using IM naloxone – statistically the same. Fast forward to 2014 when Sabzghabaee published their study on 100 patients where they reported 100% awakening with 0.4 mg IN naloxone – a better result than the 60% awakening with IV naloxone. This seemed too good to be true and not in line with almost ALL studies of naloxone where a 90% awakening +/- 5% is more typical (some patients just don’t wake up due to high levels of opiate or co ingestion of other sedatives). This study by Dietze is more in line with the Kelly data – they used 0.8 mg intranasally and found 77% arousal with one dose. So generic IV less concentrated naloxone is fairly effective when given nasally, but not as effective as the same dose given intramuscularily. If you ONLY have this lower dose and you cannot give it IM, then certainly if indicated you should use it nasally. However there are better nasal options including at least two commercially available IN naloxone products (2 and 4 mg in 0.1 ml prepackaged with an atomizer) and the original study drug – 2 mg/2 ml concentration. You will need to do your due diligence and price investigation to decide, but if prices are similar the commercially available drugs are easier to use and probably very slightly more effective in situations where we are seeing more synthetic opiate (fentanyl etc) overdoses.
Study is free open access - click here for link
Zuckerman et al describe a case of failed nasal naloxone and for some reason therefore conclude that nasal naloxone is not a reliable treatment modality for opioid overdose.[44] [It is not clear why this is reportable or why they make this conclusion. IV naloxone is also ineffective after the first dose 10-15% of the time due to the quantity of opioid consumed. As is very clear in all the provided references on this web page - there is NO 100% reliable single dose delivery method for naloxone. The providers must also support ventilation and if naloxone fails after one dose (given adequate time to be effective) they need to consider redosing, starting an IV and giving IV naloxone (only required 5-10% of the time) and seriously consider whether there is another cause for the obtundation (mixed overdose, different clinical cause such as intracranial pathology, sepsis, etc). Don't throw the baby out with the bathwater based on a single case.]
Mahonski describes the trend where increasing
availability of synthetic opiates like fentanyl has led to the need to
use higher doses of IN naloxone to reverse opioid toxicity. They found
that effective IN doses increased
from 2.1 mg in 2015 to 3.6 mg in 2017 with a concomitant decrease in
efficacy from 82% down to 76%.
In 2019 US national EMS directors published their
recommendations regarding prehospital use of naloxone. In a nutshell
they believe nasal naloxone is first line and superior to intramuscular
and if an IV is available, then IV titration is optimal.
Take away lessons for nasal drug delivery in the emergency medical setting [10]
The information provided by these studies is important in terms of needle stick risk reduction. Accidental needle sticks resulting from a patient who is an IV drug abuser are emotionally draining for the employee as well as his family. In addition, the medications used for post-exposure prophylaxis for HIV are expensive and frequently result in major side effects.[20] By administering naloxone intranasally, needle stick risk can be reduced. This improves the safety of the work environment and eliminates the professional, personal and family turmoil that may occur should a provider incur a needle stick from an IV drug abuser.
While IN medication delivery is an exciting new method for delivering medications in the EMS setting, it is not a panacea. Being aware of limitations is an important step in appropriate utilization of this therapy. Key issues that must be addressed up front are the medication dose, volume and delivery method. Once the medication and delivery method are determined there are several other issues that will improve field experience: First, be aware of clinical situations where nasal delivery may be suboptimal. Inspect the patient’s nostrils for large amounts of mucus, blood or other problems that might inhibit absorption. If abnormalities are present, consider other routes for drug administration, as there may be an increased risk of failure. A few of the failures noted for IN naloxone administration in the Denver EMS study was due to the presence of epistaxis in the patient. [13] Second, deliver the medication without delay to allow time for effective absorption. Third, relax and reassess for a few minutes. If the clinical problem fails to resolve with the intranasal medication consider two things: The nasal route was not effective or the diagnosis is wrong. (It is fairly clear from the literature that
the later is most likely the case - in every study that looked at it most patients receiving naloxone in any form did not have opiate overdoses.[14,23]) In situations where a comatose patient fails to awaken with naloxone, continue to support breathing and circulation, administer naloxone via the IM or IV route and consider alternate causes for the coma.Personal insights from experienced clinicians
Debra Kerr, PhD Candidate, Senior Fellow – Emergency Medicine Research, Melbourne Australia ….. Rapid depression of the syringe (to atomize the drug out of the ato
miser) is important and avoids respiratory administration. For paramedic use, response times may affect acceptability of IN administration as first line medication. Delay in clearance from overdose scene may reduce response times to next patient. Naloxone is not currently manufactured in a form suitable for IN administration. We had the drug manufactured by a private pharmaceutical company for the purpose of the (recent) trial. Also, the drug is not approved for IN administration by Australian legislative authorities. Anecdotally, paramedics are very keen to administer Naloxone via the IN route to reduce BBV transmission risk. Acceptability for rousable patients has not been tested. Our studies only included unrousable patients.Erik D. Barton, MD, MS, MBA; Chief of Emergency Medicine, The University of Utah, Salt Lake City….. The biggest benefit we can offer any provider who is trying to care for IVDU’s who accidentally overdose on injected opiates is safety from blood exposure. Blood-borne exposures can be both physically and emotionally devastating to non-abusers (and their families!) who were just trying to save a life. There is often a period of several months to years in which monitoring for hepatitis and HIV seroconversion must occur. The IN route offers an immediate, noninvasive, and nearly risk-free opportunity to intervene with these patients by any first-responder: family members, police, fire, EMS, and even the ED. There is no downside to attempting such a noninvasive maneuver in a suspected IVDU FIRST as long as other resuscitative efforts are not significantly delayed, especially when the benefits to the patient and provider, as described above, significantly outweigh the risks.
Tim Wolfe, MD, emergency medicine specialist, prior academician (Associate professor, University of Utah, Salt Lake City), inventor of the MAD mucosal atomization device.... Interestingly, the mean time from heroin injection to death in fatal overdoses is 60-70 minutes. This tells us that the drug itself is only part of the issue in the final apneic cardiac arrest, otherwise they would die in 5-10 minutes. I suspect, as do others who taught me this, that opiate induced respiratory depression leads to hypercarbia (high CO2 in the blood due to reduced respiratory rate). This in turn leads to further suppression of the ventilatory drive, further hypercarbia and eventually such severe hypoxia that the patient arrests. The point of course is the importance of supportive ventilation which alone may lead to patient arousal without naloxone. As emergency providers we can get very excited and impatient with nasal naloxone, expecting instant results from a drug we provide (we are driven by the unknown, fears of failure and adrenaline responses). My suggestion - relax, begin bag ventilation (reduces their hypercarbia), administer the nasal naloxone and be patient. It takes 3-5 minutes for any effect (note that IV naloxone takes about 3 minutes after administration - and it requires the time to start an IV) and up to 10 minutes for awakening. Generally they are not as agitated with nasal naloxone (probably due to less hypoxia when they finally awaken) and sometimes they are not fully awake- just breathing which is really our primary goal.
NOVEMBER 2015 news flash: FDA approves intranasal naloxone for both prescription and probably OTC use:
Adapt Pharma company announced formal FDA approval of a 4 mg/0.1 ml single dose, single nostril formulation of naloxone - NARCAN Nasal spray. They also acquired the trademark brand name "Narcan." Sales are expected to begin in the USA early in 2016. They have special pricing for first responders and community based treatment programs of $37.50 per preloaded device.
Click here for the company announcement
Editorial comment: Even though I invented the MAD nasal, began the research on nasal naloxone in the 1990s and have used this therapy for 18 years (so have a bit of a historical bent towards the original method of delivery), it seems pretty apparent to me that this new product is probably a better method for delivery of nasal naloxone than the way we have posted here on this website for the last 7 years. The new formulation is more appropriately concentrated, it has a pre-attached atomizer and because of the recent price increased in generic naloxone (single supplier cranked the price last year) this new formulation is not only better formulated, its also less expensive (in 2016). If they continue pricing it properly they should replace all generic "home made" kit formulations, if not - as of late 2017 - the research data does not show any better results than less concentrated generic formulations so the end user will need to decide which formulation to choose based on budget and convenience issues. I hope the company keeps this life saving drug at a reasonable price.
Treatment protocol
Indications:
For use on patients suspected of opiate overdoseProcedure:
If no arousal occurs after 5-10 minutes, proceed down standard unconscious protocol including injectable naloxone and secure airway if necessary.
Comment: Most "failures" of IN naloxone are due to being in a hurry to see the patient wake up. IN naloxone takes 3-5 minutes to begin working. The patients often just breath but do not come up crazy so do not always expect full arousal. (The goal is breathing)
Comment 2: IN naloxone is now being used at a BLS, law enforcement and layperson level with good success.
Teaching materials:
Intranasal naloxone slide show presentations
Wolfe - Intranasal naloxone in safe injection setting
Dyer, Snuffing out the overdose: The Boston BLS nasal naloxone program (11 MB)
Gleghorn, A brief history of overdose prevention in San Francisco 2012
Intranasal Naloxone (Narcan) articles:
Doe-Simkins, Bystander Administered Naloxone, Am J Public Health 2009.pdf
Kim, Expanded access to naloxone, Am J Public Health 2009
Dasgupta, Opioid OD - a prescription for harm prevention, Am J Lifestyle Med 2009
Leavitt, Intranasal naloxone for at home opioid rescue, Practical Pain Managment October 2010
New York City Intranasal Naloxone rescue kit directions
MMWR Feb 17 2012: Community based naloxone programs
Community based opioid overdose prevention programs, MMWR 2012
Wagner, K.D., et al., "I felt like a superhero": the experience of responding to drug overdose among individuals trained in overdose prevention. Int J Drug Policy, 2014. 25(1): p. 157-65. - go to the end of the series for this document
Hammet, Pharmacies as providers of expanded health access for PWID, BMC Health 2014 Open access
Acute opiate/heroin overdose: Intranasal therapy in EMS teaching document and quiz
Nasal Naloxone (Narcan) for pre-hospital opiate overdose - teaching document (click here) (MS Word 0.12 MB)
Nasal Naloxone (Narcan) for pre-hospital opiate overdose - Quiz (click here) (MS Word 0.05MB)
Nasal Naloxone (Narcan) for pre-hospital opiate overdose - Quiz answer key (click here) (MS Word 0.03 MB)
Other links of interest regarding IN naloxone
Audio interview with Debra Kerr - IN naloxone researcher
Project Lazarus - Home IN naloxone in North Carolina
Boston Massachusetts experience with home naloxone
Updated report on Massachusetts layperson trial (saving 755 lives to date) 2011
Boston Massachusetts experience with BLS delivered naloxone 2006
Boston Massachusetts experience with BLS naloxone 2005-2009
Legality of prescribing home naloxone in North Carolina
Leavitt, Intranasal naloxone: Overcoming opiate overdose
National public radio - Overdose rescue kits save lives
Expanding Naloxone availability in Australia (Many other links from this site)
Time Magazine - Lifesaving Overdose antidote should be made more widely available 2012 article
Open society Foundation white paper on IN naloxone 2012
Harm Reduction Coalition document describing how to create naloxone rescue kits for the lay person
MMWR paper on community opioid overdose prevention
Home naloxone treatment with intramuscular injection now FDA approved - EVIZIO - (Editor note: Expensive and still a needle stick risk, but better than no treatment)
James Roberts Emergency Medicine news article on BLS naloxone Sept 2014
Maryland toxicology report on layperson naloxone Oct 2014
Nasal Naloxone price set to Jump (Fall 2014)
http://www.theverge.com/2015/9/25/9398823/naloxone-heroin-overdose-cvs-stores-over-the-counter
http://www.huffingtonpost.com/entry/cvs-naloxone-overdose-reversal_5602dba2e4b0fde8b0d0d189
Opiate rehab clinic: The Recovery Village
RehabCenter.net Naloxone rescue kit downloadable protocol
The PDF file downloaded from their site (click here)
Bibliography (click here for abstracts)
1. Marcus, R., et al., Risk of human immunodeficiency virus infection among emergency department workers. Am J Med, 1993. 94(4): p. 363-70.
2. Kelen, G.D., et al., Hepatitis B and hepatitis C in emergency department patients. N Engl J Med, 1992. 326(21): p. 1399-404.
3. Baker, J.L., et al., Unsuspected human immunodeficiency virus in critically ill emergency patients. Jama, 1987. 257(19): p. 2609-11.
4. Osterwalder, J.J., Patients intoxicated with heroin or heroin mixtures: how long should they be monitored? Eur J Emerg Med, 1995. 2(2): p. 97-101.
5. Vilke, G.M., et al., Are heroin overdose deaths related to patient release after prehospital treatment with naloxone? Prehosp Emerg Care, 1999. 3(3): p. 183-6.
6. Smith, D.A., et al., Is admission after intravenous heroin overdose necessary? Ann Emerg Med, 1992. 21(11): p. 1326-30.
7. Hussain, A., R. Kimura, and C.H. Huang, Nasal absorption of naloxone and buprenorphine in rats. Int J Pharm, 1984. 21: p. 233-237.
8. Loimer, N., P. Hofmann, and H.R. Chaudhry, Nasal administration of naloxone is as effective as the intravenous route in opiate addicts. Int J Addict, 1994. 29(6): p. 819-27.
9. (1999) NIOSH Alert: Preventing needlestick injuries in health care settings. National Institute for Occupational Safety and Health, http://www.cdc.gov/niosh/2000-108.html#1
10. Wolfe, T.R. and E.D. Barton, Reducing needlestick risk: Nasal drug delivery in EMS. J Emerg Med Serv JEMS, 2003. 28(12): p. 52-63.
11. Marcus, R., et al., Occupational blood contact among prehospital providers. Ann Emerg Med, 1995. 25(6): p. 776-9.
12. Valenzuela, T.D., et al., Occupational exposure to hepatitis B in paramedics. Arch Intern Med, 1985. 145(11): p. 1976-7.
13. Pepe, P.E., et al., Viral hepatitis risk in urban emergency medical services personnel. Ann Emerg Med, 1986. 15(4): p. 454-7.
14. Barton, et al., Efficacy of intranasal naloxone as a needleless alternative for treatment of opioid overdose in the prehospital setting. J Emerg Med, 2005. 29(3): p. 265-71.
15. Kelly, et al., Randomised trial of intranasal versus intramuscular naloxone in prehospital treatment for suspected opioid overdose. Med J Aust, 2005. 182(1): p. 24-7.
16. Martin, T.G., Take home naloxone: feasability, safety and efficacy. J Toxicol Clin Toxicol, 2003. 41(4): p. 415-416.
17. Belz, D., et al., Naloxone use in a tiered-response emergency medical services system. Prehosp Emerg Care, 2006. 10(4): p. 468-71.
18. Baca, C.T. and K.J. Grant, Take-home naloxone to reduce heroin death. Addiction, 2005. 100(12): p. 1823-31.
19. Wanger, K., et al., Intravenous vs subcutaneous naloxone for out-of-hospital management of presumed opioid overdose. Acad Emerg Med, 1998. 5(4): p. 293-9.
20. Parkin, J.M., et al., Tolerability and side-effects of post-exposure prophylaxis for HIV infection. Lancet, 2000. 355(9205): p. 722-3.
21. Robertson, T.M., et al., Intranasal naloxone is a viable alternative to intravenous naloxone for prehospital narcotic overdose. Prehosp Emerg Care, 2009. 13(4): p. 512-5.
22. Doe-Simkins, M., et al., Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am J Public Health, 2009. 99(5): p. 788-91.
23. Merlin M.A., Saybolt M., Kapitanyan R, et al (2009) "Intranasal naloxone delivery is an alternative to intravenoius naloxone for opioid overdoses." Am J Emerg Med - published online Oct 2009, pending journal publication
24. Kerr, D., A. M. Kelly, et al. (2009). "Randomized controlled trial comparing the effectiveness and safety of intranasal and intramuscular naloxone for the treatment of suspected heroin overdose." Addiction 104(12): 2067-74.
25. Wermeling, D. P. (2010). "Opioid harm reduction strategies: focus on expanded access to intranasal naloxone." Pharmacotherapy 30(7): 627-631.
26.
McDermott, C. and N. C. Collins (2012). "Prehospital medication
administration: a randomised study comparing intranasal and intravenous
routes." Emerg Med Int
2012: 476161.
28. Unick GJ, Rosenblum D, Mars S, Ciccarone D (2013) Intertwined Epidemics: National Demographic Trends in Hospitalizations for Heroin- and Opioid- Related Overdoses, 1993–2009. PLoS ONE 8(2): e54496. doi:10.1371/journal.pone.0054496
29. Coffin, P. O. and S. D. Sullivan (2013). "Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal." Ann Intern Med 158(1): 1-9.
30.Compton,
W. M., N. D. Volkow, et al. (2013). "Expanded access to opioid overdose
intervention: research, practice, and policy needs." Ann Intern Med
158(1): 65-66.
50. Rando, J., D. Broering, et al. (2015). "Intranasal naloxone
administration by police first responders is associated with decreased
opioid overdose deaths." Am J Emerg Med.
51. Fareed, A., A. M. Buchanan-Cummings, et al. (2015). "Reversal of
overdose on fentanyl being illicitly sold as heroin with naloxone nasal
spray: A case report." Am J Addict
24(5): 388-390.
52. Dwyer, K., A. Y. Walley, et al. (2015). "Opioid education and nasal
naloxone rescue kits in the emergency department." West J Emerg Med
16(3): 381-384.
53. Dahlem, C. H., M. J. Horstman, et al. (2015). "Development and
implementation of intranasal naloxone opioid overdose response protocol
at a homeless health clinic." J Am Assoc Nurse Pract.
54.
Fisher, R., D. O'Donnell, et al. (2016). "Police Officers Can Safely and
Effectively Administer Intranasal Naloxone." Prehosp Emerg Care:
1-6.
55. Akers, J. L., R. N. Hansen, et al. (2017). "Implementing take-home
naloxone in an urban community pharmacy." J Am Pharm Assoc (2003)
57(2S): S161-S167.
57. Chang, G., M. Davids, et al. (2017). "Overdose
education and naloxone distribution for veterans with opioid use
disorder: Results from a pilot initiative." J Addict Dis
36(4): 217-221.
58. Heavey, S. C., A. M. Delmerico, et al. (2017). "Descriptive
Epidemiology for Community-wide Naloxone Administration by Police
Officers and Firefighters Responding to Opioid Overdose." J Community
Health.
59. Kobayashi, L., T. C. Green,
et al. (2017). "Patient Simulation for Assessment of Layperson
Management of Opioid Overdose With Intranasal Naloxone in a Recently
Released Prisoner Cohort." Simul Healthc
12(1): 22-27.
60. Han, J. K., L. G. Hill, et al. (2017). "Naloxone Counseling for Harm
Reduction and Patient Engagement." Fam Med
49(9): 730-733.
61. Gulec, N., J. Lahey, et al. (2017). "Basic and
Advanced EMS Providers Are Equally Effective in Naloxone Administration
for Opioid Overdose in Northern New England." Prehosp Emerg Care:
1-7.
62. Klebacher, R., M. I. Harris, et al. (2017). "Incidence of Naloxone
Redosing in the Age of the New Opioid Epidemic." Prehosp Emerg Care:
1-6.
63. Weiner, S. G., P. M.
Mitchell, et al. (2017). "Use of Intranasal Naloxone by Basic Life
Support Providers." Prehosp Emerg Care
21(3): 322-326.
64.
Rosenberg, M., G. Chai, et al. (2018). "Trends and economic drivers for
United States naloxone pricing, January 2006 to February 2017."
Addict Behav 86: 86-89.
65. Dietze, P., M. Jauncey, et al. (2019). "Effect of Intranasal vs
Intramuscular Naloxone on Opioid Overdose: A Randomized Clinical Trial."
JAMA Netw Open 2(11):
e1914977.
66. Guadamuz, J. S., G. C. Alexander, et al. (2019). "Availability and
Cost of Naloxone Nasal Spray at Pharmacies in Philadelphia,
Pennsylvania, 2017." JAMA Netw Open
2(6): e195388.
67. Wu, C., T. Brown, et al. (2019). "Access to
naloxone at community pharmacies under the Massachusetts statewide
standing order." J Am Pharm Assoc (2003).
68. Krieter, P. A., C. N. Chiang, et al. (2019). "Comparison of the
Pharmacokinetic Properties of Naloxone Following the Use of FDA-Approved
Intranasal and Intramuscular Devices Versus a Common Improvised Nasal
Naloxone Device." J Clin Pharmacol
59(8): 1078-1084.
70. Pruyn, S., J. Frey, et al. (2019). "Quality Assessment of Expired
Naloxone Products from First-Responders' Supplies." Prehosp Emerg
Care 23(5): 647-653.
71. Skulberg, A. K., A. Asberg, et al. (2019). "Pharmacokinetics of a
novel, approved, 1.4-mg intranasal naloxone formulation for reversal of
opioid overdose-a randomized controlled trial." Addiction
114(5): 859-867.
72. Smart, R., C. K. Geiger, et al. (2019). "An
Observational Study of Retail Pharmacy Naloxone Prescriptions:
Differences Across Provider Specialties and Patient Populations." J
Gen Intern Med.
73. Strang, J., R. McDonald, et
al. (2019). "Take-Home Naloxone for the Emergency Interim Management of
Opioid Overdose: The Public Health Application of an Emergency
Medicine." Drugs 79(13): 1395-1418.
74. Tippey, K. G., M. Yovanoff, et al. (2019). "Comparative Human
Factors Evaluation of Two Nasal Naloxone Administration Devices:
NARCAN((R)) Nasal Spray and Naloxone Prefilled Syringe with Nasal
Atomizer." Pain Ther 8(1):
89-98.
75. Williams, K., E. S. Lang, et al. (2019). "Evidence-Based Guidelines
for EMS Administration of Naloxone." Prehosp Emerg Care
23(6): 749-763.
 Therapeutic
Intranasal Drug Delivery
Therapeutic
Intranasal Drug Delivery