Intranasal glucagon for severe hypoglycemia
Table of Contents:
Introduction to nasal glucagon (click here)
Literature overview and discussion (click here)
Personal insights from experienced clinicians (click here)
Treatment protocols (click here)
Teaching materials (click here)
Introduction
On a year to year basis, between 10% and 30% of patients on insulin therapy suffer severe hypoglycemia requiring external assistance.[1] Current treatment modalities include oral glucose (which may be poorly tolerated in the unconscious patient), intramuscular glucagon and intravenous dextrose. The later two options require injections of the medications and are difficult skills for family members to master. Therefore, emergency medical services (EMS) are frequently contacted to respond to these patients and to administer appropriate therapies to increase the patient’s blood sugar. If it were possible to administer glucagon intranasally without the need for an injection, family members could resuscitate their diabetic relatives much more easily and EMS calls would decrease.[2] This treatment option would also be attractive to the EMS workers themselves – reducing the risk of a needle stick in a patient population that is often confused and combative.
Literature overview and discussion
A moderate amount of literature exists demonstrating that intranasal glucagon is effective in treating hypoglycemia. Most of this literature suggests that nasal glucagon is optimally absorbed if mixed with a surfactant additive (such as sodium glycocholate rather than the sterile water diluent that comes with the package) to enhance absorption. The literature is also fairly clear in showing that intramuscular or subcutaneous glucagon leads to more rapid rises in blood glucose with longer effect.[3, 4] Pontiroli and colleagues have published the majority of data on this topic.[4-7,18] Boido et al published a meta-analysis evaluating the efficacy of glucagon versus dextrose for raising blood sugar in hypoglycemic patients and comparing glucagon given IV versus IN for the treatment of hypoglycemia.[15] They conclude that the ineffectiveness of glucagon is infrequent, not different from dextrose; in addition, intranasal and injected glucagon are similarly effective. In the case of failure, a second dose can be administered. Other authors have also confirmed the effectiveness of IN glucagon when mixed with absorptive enhancers.[8, 9, 13, 17]
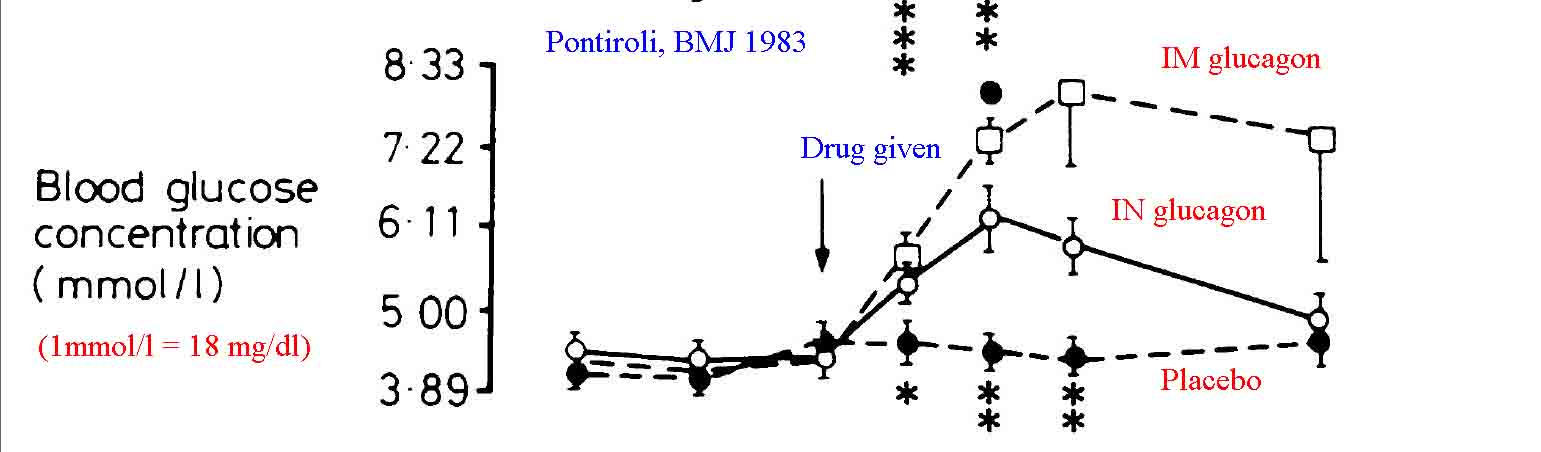
Despite the majority of published data noting improved results with a surfactant additive, there are studies that simply used standard glucagon solubilized in the packaged diluent (sterile water) as an intranasal spray and noted effective results. Hvidberg et al found that 2 mg of IN glucagon solution was effective in raising blood glucose levels of hypoglycemic patients, though not as quickly as that found with 1 mg of intramuscular glucagon.[10] Pacchioni et al found IN glucagon as effective as IV glucagon at stimulating gut motility for radiologic procedures.[11] Rosenfalck et al compared 1 mg and 2 mg doses of intranasal glucagon to 1 mg does of intramuscular glucagon in hypoglycemic adults. They found all three methods more effective at raising blood glucose than observation alone. Furthermore they found the 2 mg IN dose to be equal to the 1 mg IM dose, suggesting a potential role for intranasal glucagon in the treatment of accidental hypoglycemia, especially if higher doses were used.[12]IN 2015 Pontiroli reviewed the available literature on the effectiveness of intranasal glucagon, concluding that it is a promising alternative to more traditional methods of delivery.[18]
Numerous discussions exist on the Internet demonstrating that paramedics are using intranasal glucagon in unconscious patients with hypoglycemia. These discussions suggest that this method is of interest to EMS workers, but as of mid 2008 no published data exists to demonstrate the results of their experiences.
2012 - The first EMS study was published in the fall of 2012 describing the use of intranasal glucagon in the EMS setting.[14] This article describes a case of a woman who was unconscious and had a blood sugar of 21. After 3 failed attempts they administered 1 mg of IN glucagon and she aroused fairly quickly. Repeat blood sugar as 116. Though this is really the first published EMS case describing successful use in IN glucagon, the authors provide an in-depth review of the other literature relating to intranasal and intramuscular glucagon. They conclude that the drug is effective intranasally but considerable more expensive than IV glucose. However in a setting such as BLS response or rural EMS response the benefits of IN glucagon in the hands of a BLS provider probably far outweigh the additional costs of accessing ALS care. Furthermore they provide evidence that the lay public would appreciate a nasal glucagon kit were it available – again justifying the cost to allow families to treat hypoglycemic spells.
2016: Rickels et al studied IN (3 mg) versus IM glucagon (1 mg)
and found them similarly effective at reversing hypoglycemia in a
controlled setting with onset of action in 16 vs 13 minutes
respectively, and success defined as plasma glucose over 70 mg/dl in
98.7 vs 100% of cases.[19] Phase III trials are also beginning for a
commercially available form
of IN glucagon - results pending.
(click here for pharma company data regarding IN glucagon)
2016:
Sherr et al report the phase 1 trial results comparing IN glucagon powder
to IM glucagon.[20] They conducted their trial using children 4 to 17
years of age. They also find both methods highly effective at reversing
insulin induced hypoglycemia.
By 2020 the pharma industry had caught on and there is now a branded form of nasal glucagon on the market. Further more, many additional studies have been published supporting its efficacy. [21-29]
In summary, intranasal glucagon is effective for the treatment of hypoglycemia, especially if it is solubilized with an absorptive enhancer such as sodium glycocholate. It is less clear how effective IN glucagon is when solubilized in sterile water though there are increasing reports from EMS providers that it is effective. In the current packaging, which uses sterile water as the solubilization medium, several small studies do suggest intranasal glucagon is superior to placebo when given in doses of 1-2 mg. There may be a role for this medication (as intranasal formulation) in home therapy, BLS settings and in combative patients with severe hypoglycemia since it can be given without an injection – an advantage that would likely enhance its use in the home setting.[2] It would be nice to see this role confirmed with more research data from EMS agencies. Even a simple retrospective chart review proving that administration of this therapy led to an increase in serial blood sugars would be stronger real world clinical evidence than anything published to date (Nov 2014).
Personal insights from experienced clinicians
Brian Laura Pulley, EMT-P Chesterfield Virginia
Editorial comment -- this is an "n" of one so can't be considered definitive data but it is insightful and suggests what we have commented above - this area of IN delivery needs more clinical research by medics and emergency personnel. IN fentanyl, naloxone and midazolam are supported by many many trials, but we still need more data on IN glucagon before routine adoption (if at all).
This section awaits more user input - if anyone has personal experience and is willing to contribute please e-mail info@intranasal.net and be patient since we only check it every month or so as we update this web site. We would love some personal insight and input as we continue to hear from the EMS community how useful this is and wonder if it should be implemented at home so EMS is not even called.
Treatment protocol
Indications: For use on patients with documented hypoglycemia
Procedure:
- Assess ABC’s – Airway, Breathing, Circulation
- For pulseless patients, proceed to ACLS guidelines
- If hypoxemia or apnea exists– Establish oral airway and begin bag ventilation with 100% oxygen
- Check finger stick blood glucose.
- Consider other rapidly reversible causes of coma (opiate overdose, hypoxemia)
- If hypoglycemia is documented by finger stick blood glucose, continue as below:
- Solubilize 2 mg of glucagon (2 vials) in a total volume of 1 ml of diluent (1/2 ml per vial). If possible obtain sodium glycocholate as the diluent, if not use that which comes with the package.
- Load syringe with 2 mg of glucagon and attach a nasal atomizer.
- Place atomizer within the nostril
- Briskly compress syringe to administer 1/2 of atomized spray.
- Remove and repeat in other nostril, so the entire 2 mg of medication are administered.
- Simultaneously administer glucose paste under lip and tongue to further enhance blood sugar elevation.
- Continue ventilating patient as needed
Teaching materials
None at this time, please submit any personal insights you have to be posted here on this web site. Please e-mail info@intranasal.net and be patient since we only check it every month or so as we update this web site. We would love some personal insight and input as we continue to hear from the EMS community how useful this is and wonder if it should be implemented at home so EMS is not even called.
It would be nice to see this role confirmed with more research data from EMS agencies. If you have implemented this treatment module (IN glucagon) in your EMS setting this is a very easy study that would be published. Even a simple retrospective chart review proving that administration of this therapy led to an increase in serial blood sugars would be stronger real world clinical evidence than anything published to date (as of Nov 2014).
Links of interest
Bibliography (click here for abstracts)
1. Carstens, S. and I. Andersen, [Intranasal glucagon in the treatment of hypoglycemia. A therapeutic possibility in the future]. Ugeskr Laeger, 1994. 156(30): p. 4339-42.
2. Yanai, O., et al., IDDM patients' opinions on the use of glucagon emergency kit in severe episodes of hypoglycemia. Practical Diabetes, 2005. 14(2): p. 40-42.
3. Stenninger, E. and J. Aman, Intranasal glucagon treatment relieves hypoglycaemia in children with type 1 (insulin-dependent) diabetes mellitus. Diabetologia, 1993. 36(10): p. 931-5.
4. Pontiroli, A.E., et al., Nasal administration of glucagon and human calcitonin to healthy subjects: a comparison of powders and spray solutions and of different enhancing agents. Eur J Clin Pharmacol, 1989. 37(4): p. 427-30.
5. Pontiroli, A.E., M. Alberetto, and G. Pozza, Metabolic effects of intranasally administered glucagon: comparison with intramuscular and intravenous injection. Acta Diabetol Lat, 1985. 22(2): p. 103-10.
6. Pontiroli, A.E., et al., Intranasal glucagon as remedy for hypoglycemia. Studies in healthy subjects and type I diabetic patients. Diabetes Care, 1989. 12(9): p. 604-8.
7. Pontiroli, A.E., et al., Pharmacokinetics of intranasal, intramuscular and intravenous glucagon in healthy subjects and diabetic patients. Eur J Clin Pharmacol, 1993. 45(6): p. 555-8.
8. Freychet, L., et al., Effect of intranasal glucagon on blood glucose levels in healthy subjects and hypoglycaemic patients with insulin-dependent diabetes. Lancet, 1988. 1(8599): p. 1364-6.
9. Slama, G., et al., A new non-invasive method for treating insulin-reaction: intranasal lyophylized glucagon. Diabetologia, 1990. 33(11): p. 671-4.
10. Hvidberg, A., R. Djurup, and J. Hilsted, Glucose recovery after intranasal glucagon during hypoglycaemia in man. Eur J Clin Pharmacol, 1994. 46(1): p. 15-7.
11. Pacchioni, M., et al., The hypotonic effect of intranasal and intravenous glucagon in gastrointestinal radiology. Abdom Imaging, 1995. 20(1): p. 44-6.
12. Rosenfalck, A.M., et al., Nasal glucagon in the treatment of hypoglycaemia in type 1 (insulin-dependent) diabetic patients. Diabetes Res Clin Pract, 1992. 17(1): p. 43-50.
13.
Nakazato, M. (2011). "[Development of the novel delivery system of GLP-1
administration for the treatment of diabetes mellitus]." Nihon Rinsho
69(5): 918-922.
14.
Sibley, T., R. Jacobsen, et al. (2012). "Successful Administration of
Intranasal Glucagon in the Out-of-Hospital Environment." Prehosp
Emerg Care.
18. Pontiroli AE. Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. Journal of diabetes science and technology 2015;9:38-43.
19.
Rickels, M. R., K. J. Ruedy, et al. (2016). "Intranasal Glucagon for
Treatment of Insulin-Induced Hypoglycemia in Adults With Type 1
Diabetes: A Randomized Crossover Noninferiority Study." Diabetes Care
39(2): 264-270.
20. Sherr, J. L., K. J. Ruedy, et al. (2016). "Glucagon Nasal Powder: A Promising Alternative to Intramuscular Glucagon in Youth With Type 1 Diabetes." Diabetes Care 39(4): 555-562.
 Therapeutic
Intranasal Drug Delivery
Therapeutic
Intranasal Drug Delivery